| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
1
H
1.0079
|
2
He
4.0026
|
||||||||||||||||
| 2 |
3
Li
6.94
|
4
Be
9.0121
|
5
B
10.81
|
6
C
12.011
|
7
N
14.007
|
8
O
15.999
|
9
F
18.9984
|
10
Ne
20.1797
|
||||||||||
| 3 |
11
Na
22.9898
|
12
Mg
24.305
|
13
Al
26.9815
|
14
Si
28.085
|
15
P
30.9738
|
16
S
32.06
|
17
Cl
35.45
|
18
Ar
39.949
|
||||||||||
| 4 |
19
K
39.0983
|
20
Ca
40.078
|
21
Sc
44.9559
|
22
Ti
47.867
|
23
V
50.9415
|
24
Cr
51.9961
|
25
Mn
54.938
|
26
Fe
56.845
|
27
Co
58.9332
|
28
Ni
58.6934
|
29
Cu
63.546
|
30
Zn
65.38
|
31
Ga
69.723
|
32
Ge
72.63
|
33
As
74.9216
|
34
Se
78.971
|
35
Br
79.904
|
36
Kr
83.798
|
| 5 |
37
Rb
85.4678
|
38
Sr
87.62
|
39
Y
88.9058
|
40
Zr
91.224
|
41
Nb
92.9064
|
42
Mo
95.95
|
43
Tc
98
|
44
Ru
101.07
|
45
Rh
102.906
|
46
Pd
106.42
|
47
Ag
107.868
|
48
Cd
112.414
|
49
In
114.818
|
50
Sn
118.71
|
51
Sb
121.76
|
52
Te
127.6
|
53
I
126.904
|
54
Xe
131.293
|
| 6 |
55
Cs
132.905
|
56
Ba
137.327
|
La-Lu
|
72
Hf
178.49
|
73
Ta
180.948
|
74
W
183.84
|
75
Re
186.207
|
76
Os
190.23
|
77
Ir
192.217
|
78
Pt
195.084
|
79
Au
196.967
|
80
Hg
200.592
|
81
Tl
204.38
|
82
Pb
207.2
|
83
Bi
208.980
|
84
Po
(209)
|
85
At
(210)
|
86
Rn
(222)
|
| 7 |
87
Fr
(223)
|
88
Ra
(226)
|
Ac-Lr
|
104
Rf
(267)
|
105
Db
(270)
|
106
Sg
(269)
|
107
Bh
(270)
|
108
Hs
(270)
|
109
Mt
(278)
|
110
Ds
(281)
|
111
Rg
(281)
|
112
Cn
(285)
|
113
Nh
(286)
|
114
Fl
(289)
|
115
Mc
(289)
|
116
Lv
(293)
|
117
Ts
(293)
|
118
Og
(294)
|
| 6 |
57
La
138.905
|
58
Ce
140.116
|
59
Pr
140.908
|
60
Nd
144.242
|
61
Pm
145
|
62
Sm
150.36
|
63
Eu
151.964
|
64
Gd
157.25
|
65
Ib
158.925
|
66
Dy
162.5
|
67
Ho
164.930
|
68
Er
167.259
|
69
Tm
168.934
|
70
Yb
173.045
|
71
Lu
174.967
|
|||
| 7 |
89
Ac
(227)
|
90
Th
232.038
|
91
Pa
(231)
|
92
U
238.029
|
93
Np
(237)
|
94
Pu
(244)
|
95
Am
(243)
|
96
Cm
(247)
|
97
Bk
(247)
|
98
Cf
(251)
|
99
Es
(252)
|
100
Fm
(257)
|
101
Md
(258)
|
102
No
(259)
|
103
Lr
(262)
|
Basic Data I
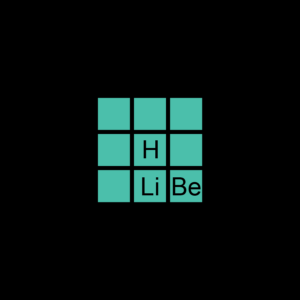
| Atomic Number | 1 |
| Atomic Symbol | H |
| Atomic Weight | 1.0079 |
| Natural occurance | Primordial |
| Color | Colorless |
| Group | 1 |
| Period | 1 |
| Family | Lithium |
| Block | S |
Basic Data II
| Classification | Nonmetal |
| Phase at 25C | Gas |
| Density at 25C | 0.0000899 g/cm3 |
| Atomic volume | 14.4 cm3/mol |
| Hardness | – |
| Melting Point | 14.01 K |
| Boiling Point | 20.28 K |
| Cost, pure | $12 per 100g |
| Cost, bulk | – |
Abundance
Parts per billion in atoms
| Universe | 930000000 |
| Sun | 930000000 |
| Meteorites | 170000000 |
| Earth’s crust | 31000000 |
| Oceans | 662000000 |
| Humans | 620000000 |
History
| Discoverer | Henry Cavendish |
| Year Discovered | 1766 |
| Place Discovered | United Kingdom |
| First Isolated | 1671 |
| Isolated By | Robert Boyle |
Uses
| Ammonia Production | |
| Fuel Cells | |
| Hydrogenation | |
| Pharmaceuticals | |
| Power Generation | |
| Rocket fuels | |
| Semiconductors |
Atomic Properties
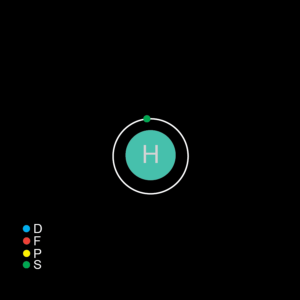
| Electrons | 1 |
| Neutrons- most abundant isotope | 0 |
| Protons | 1 |
| Shell structure | 1 |
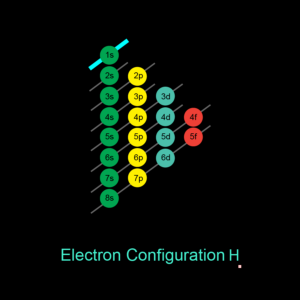
| Electron Configuration | 1s1 |
| Oxidation States | -1,1 |
Atomic Structure
| Atomic Radius Calculated | 53 pm |
| Atomic Radis Empirical | 25 pm |
| Atomic Volume | 22.4135 cm3/mol |
| Covalent Radius | 37 pm |
| Van der Waals Radius | 120 pm |
| Neutron Cross Section | 0.332 |
| Neutron Mass Absorption | 0.011 |
Other Info
| Electrical conductor | ? |
| Conductivity | N/A |
| Resistance | N/A |
| Magnetism | Diamagnetic |
| Thermal Conductivity | 0.1805 W/(m K) |
| Thermal Expansion | N/A |